Corrosion know-how facilitates the use of hydrogen as the fuel of tomorrow
Fossil fuels are nearing extinction. Electrolyzer, a plausible solution?
Brussels (pts007/13.12.2021/09:00) As we all know, fossil fuels are finite and experts are working tirelessly to find sustainable sources of alternative energy that will fuel the world of tomorrow. Hydrogen gas has been considered a valid option for the past decade since it can be used to generate electricity via fuel cells through a process of turning hydrogen gas into water. The main issue was that hydrogen is mainly sourced from natural gas. Since natural gas is indeed a fossil fuel, it is not sustainable in the long term.
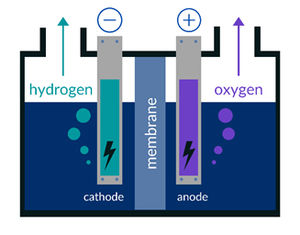
As the name suggests, an electrolyzer is a system that enables the process of electrolysis. This technique powers an otherwise non-spontaneous chemical reaction with direct electric current. 'Electrolysis' means literally 'breakdown by electricity', hence it uses electricity to separate water into both hydrogen and oxygen (2H2O > 2H2 + O2).
A single electrolyzer cell is not feasible since it has a very limited output in gas volumes/gas volume rates. To upscale this process the single stacks are repeated several times to produce an electrolyzer stack (or idem fuel cell stack). Some plates have a dual purpose, being both the cathode and the anode hence they are called 'bipolar plates'. Bipolar plates are equipped with small flow channels to transport the gas and water.
Since our blue planet consists of 71% water and the electrolyzer does not require the input of fossil fuels, this might be an interesting path to pursue in the search for more durable sources of energy. As mentioned above a fuel cell creates electrical energy by doing the exactly opposite process. Thus creating a cycle.
Logistical challenge
Our planet is equipped with a network of supply chains to carry fossil fuels from point A to B. Some houses have a central heating system on natural gas. The gas is distributed from the energy provider to the consumer via physical pipes.
For example, imagine your home is equipped with a gas burner as a means to facilitate domestic heating. A domestic gas burner can be adapted to burn hydrogen gas instead of natural gas. The hydrogen gas still needs to reach the consumer.
The solution seems straightforward: use the natural gas pipes to transport hydrogen gas instead. Here comes the first challenge. Hydrogen gas has different properties than natural gas. The pipes are specifically created to transport natural gas and since they are susceptible to failure mechanisms specific for hydrogen, this causes the pipes to not be as suitable to transport hydrogen. Hydrogen can cause embrittlement of the material and results in a higher risk of accidental release of hydrogen gas through fracture.
A solution might be simple: skip the transportation phase and create hydrogen gas with an electrolyzer at the location where it will be used. For instance it can be used to store excess production of green energy. However even the latest designs are still too small to provide a whole house with the needed supply of hydrogen to generate electricity. The main culprit is the membrane, which proves a real challenge to be upscaled to fit an economically feasible electrolyzer to be used on consumer, let alone industrial, scale.
Lastly the bipolar metal plates that are submerged in severely acid or alkaline conditions are prone to corrosion. When corrosion occurs on the plates, the efficiency of the electrolyzer will decrease.
This challenge is especially prominent for electrolyzers since the conditions of fuel cells are less aggressive for the metal. In the following paragraphs we will elaborate on the specific approach for materials selection for fuel cells and electrolyzers
Corrosion in fuel cells: steel vs. titanium
Even though fuel cells offer a less extreme environment for the metal plates than electrolyzers, corrosion is still a delicate yet important matter in fuel cells. The allowed corrosion rate should be in the range of µm/year, this is the result of the low tolerance towards corrosion since it has a direct negative effect on the performance.
When we take a closer look at the materials that are used in fuel cells, generally high-alloy stainless steels (such as superduplex, superaustenitic ) or titanium are used. Although titanium has beneficial properties for use as base material in fuel cells, hydrogen does react with titanium. This reaction results in the formation of titanium hydride (TiH2) which causes the base material to be more brittle. When titanium is chosen in fuel cells, extra measures are taken against hydrogen embrittlement by applying surface coatings like titanium nitride.
Corrosion in electrolyzers: material selection is key
Materials selection in electrolyzers proves to be more tricky. On one hand, an electrolyzer requires a chemically resistant material that can withstand the corrosive environment. But on the other hand, the material needs to be stackable, and thus malleable. This creates a new challenge: in order to be deformable, the material should be thin which in turn reduces its service life in case corrosion occurs. A possible solution lies again in applying a thin, conductive protective layer (such as titanium nitride or platinum).
Tests to ensure you chose the right material
Research with regard to materials selection is ongoing. You may wonder where to start when choosing the right material and the extra measures that might come along in protection against corrosion.
To conduct this comparative analysis between different options, it is commendable to conduct electrochemical research, such as cyclic polarization tests with Tafel analysis. This kind of measurement is performed by means of a potentiostat. The measured corrosion current density in µA/cm^2 is converted into a corrosion rate in µm/year. A benefit of this test is the flexibility: the test generally takes less than a day which makes it possible to perform the test with different solutions or materials, even ones that you have not had any experience with.
Are you interested in getting to the bottom of what material works best for you by having an electrochemical test performed? Or are you looking to equip your lab with a potentiostat? Contact METALogic - TÜV AUSTRIA - https://www.metalogic.be/en/contact
(end)
| Aussender: | METALogic - TÜV AUSTRIA Group |
| Ansprechpartner: | Erik Thomas |
| Tel.: | +32 16 39 60 00 |
| E-Mail: | Erik.Thomas@METALogic.be |
| Website: | www.METALogic.be |